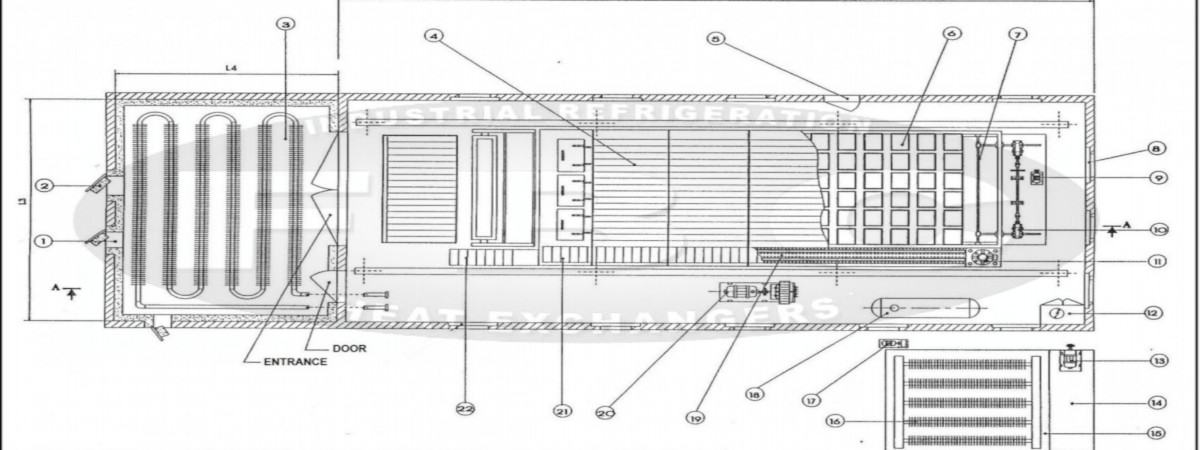
What is Anhydrous Ammonia?
You will hear a lot of people saying anhydrous ammonia is a very dangerous refrigerant.
Most of them being Freon Technicians, why? Because when people do not understand something or have not been educated or trained in a field or chemical they tend to use their gut feelings.
Yes, Ammonia smells bad and yes if released in large quantities can kill you, but if handled properly can be used safely and without danger to anyone.
Yes Ammonia has its place and is not recommended for use in large public areas unless located (engine room and storage) away from the central areas. A secondary refrigerant should be used, such as a brine or glycol mixture.
A 300 ton system does have its place but the above listed area is not one of them. The best way to make ammonia safe to use starts with safety engineering. A properly engineered system can be as safe as any refrigeration system, taking into consideration the dangers associated with this refrigerant compared to others.
In many cases Freon is a better choice because large populated areas are not as easy to control should a leak or release occur.
Anhydrous Ammonia as a refrigerant offers many benefits to its users and can and has become a safer choice simply because of better training made available to technicians, owners, plant operators and others associated with this chemical.
Properties of Ammonia
Anhydrous ammonia is the compound formed by the combination of the two gaseous elements, nitrogen and hydrogen, in the proportion of one part nitrogen and three parts of hydrogen by Volume. Since one volume of nitrogen weighs fourteen times as much as one volume of hydrogen, on a weight basis, the ration is fourteen parts of nitrogen to three parts of hydrogen, or about 82% nitrogen and 18% hydrogen.
At atmospheric temperatures and pressures, anhydrous ammonia is pungent colorless gas. Anhydrous Ammonia boils at -28F and freezes to a white crystalline mass at -107.9NF.
When heated above its critical temperature of 270.3NF ammonia exists only as a vapor regardless of the pressure. Between the melting and critical points, liquid ammonia exerts a vapor pressure which increases with rising temperature.
When liquid ammonia is in a closed container it is in equilibrium with ammonia vapor and the pressure within the container bears a definite relationship to the temperature. Ammonia liquid is lighter than water, having a density of 42.57 pounds per cubic foot at -28F, while as a vapor, ammonia is lighter than air, its relative density being 0.5970 compared to air at atmospheric pressure and temperature of 32NF.
Under the latter conditions, one pound of ammonia vapor occupies a volume of 22.5 cubic feet and yields 45 cubic feet of dissociated gas at a ration of 25% nitrogen and 75% hydrogen.
Because of its great affinity for water, care must be taken in the storage and handling of ammonia to keep it dry.
“Anhydrous” means “without water”. When ammonia gas is dissolved in water, the resulting material is called ammonium hydroxide or “aqua” ammonia. The two materials, anhydrous ammonia and aqua ammonia should not be confused.
Physical Properties of Ammonia
Molecular symbol: NH3
Molecular weight: 17.031g/mol
Boiling point at one atmosphere: -33,33°C
Freezing point at one atmosphere: -77,66°C
Critical Temperature: 132,22°C
Critical Pressure: 115,6 kg/cm²
Latent heat at-33°C (-28°F) & 1 atm: 327,1 cal/g
Relative density of vapor compared to dry air at 0°C (32°F) & 1 atm: 0,5967
Vapor density at -33°C (-28F) and 1 atm 0.8896 kg/m³
Specific gravity of liquid -33°C (-28°F) compared to water at 4°C: 0,6816
Liquid density at -33°C (28°F) & 1 atm: 681,6 kg/,³
Specific volume at vapor at 0°C (32°F) &1 atm: 1,299 m³/kg
Flammable limits by volume in air at atm pressure: 15,5%-27%
Ignition temperature: 651,1°C
This information offered as reference materials only.
HISTORY
How common is ammonia refrigeration?
Many years ago, the food and beverage industry embraced ammonia refrigeration. The economic advantages alone made it the refrigerant of choice for cold storage facilities and food processing facilities as well as the dairy and meatpacking industries. Almost all of the food on the family breakfast, lunch and dinner table passes through an ammonia refrigeration facility before reaching your grocery store including fresh fruits and vegetables, meat, poultry and fish, frozen convenience foods, milk, cheese and ice cream, and beverages such as soft drinks, beer and wine.
How long has ammonia been used as a refrigerant?
Ammonia was among the early refrigerants used in mechanical systems, and it's the only one of the early refrigerants to secure a lasting role as a refrigerant. Mechanical refrigeration was developed in the 1800s based on the principle of vapor compression. The first practical refrigerating machine using vapor compression was developed in 1834 and by the late 1800s refrigeration systems were being used in breweries and cold storage warehouses. The basic design of the vapor compressor refrigeration system, using ammonia as a refrigerant in a closed cycle of evaporation, compression, condensation, and expansion, has changed very little since the early 1900s.
When was ammonia first synthesized?
Ammonia was first synthesized in 1823 by reacting air and hydrogen. The first commercial production of synthetic ammonia began in 1913. Presently, there are an estimated two billion metric tons of ammonia present in the world. Of this amount, approximately five percent is man-made. Approximately 18 million metric tons of ammonia are produced annually in North America alone, and of this amount, less than two percent is used for refrigeration.
Why is ammonia referred to as a natural refrigerant?
Ammonia is a common, naturally occurring compound in the environment that breaks down naturally into hydrogen and nitrogen molecules (the atmosphere consists of nearly 80% nitrogen and hydrogen). Ammonia is made up of one atom of nitrogen and three atoms of hydrogen, with the chemical symbol NH3. Ammonia is a key element in the nitrogen cycle, and under normal conditions, is essential for many biological processes. Ammonia can be found in water, soil, and air, and is a source of much needed nitrogen for plants and animals. In fact, ammonia is among the most abundant gasses in the environment.
When was the first commercial use of ammonia refrigeration?
Ammonia was first used as a refrigerant in the 1850s in France and was applied in the United States in the 1860s for artificial ice production. The first patents for ammonia refrigeration machines were filed in the 1870s. By the 1900s, ammonia refrigeration machines were being commercially installed in block ice, food processing, and chemical production facilities. By the 1920s, ammonia refrigeration was being applied to ice rinks. During the 1930s, air conditioning markets began to develop, first for industrial applications and then for human comfort. The use of smaller units for domestic refrigerators increased substantially between 1920 and 1930.
How is ammonia refrigeration used today?
Ammonia refrigeration has been the backbone of the cold storage and food processing industries since the early 1900s. Ammonia refrigeration is the most cost effective and energy efficient method of processing and storing frozen and unfrozen foods. It is the workhorse for the post-harvest cooling of fruits and vegetables, the cooling of meat, poultry, and fish, refrigeration in the beverage industry, particularly for beer and wine, refrigeration of milk and cheese, and the freezing of ice cream. Practically all fruits, vegetables, produce and meats, as well as many beverages and juices, pass through at least one facility that uses an ammonia refrigeration system before reaching our homes. Ammonia refrigeration is also used in the chemical industry.
Does ammonia refrigeration have other modern day uses?
Recently, air conditioning provided by ammonia refrigeration systems has found applications on college campuses and office parks, small scale buildings such as convenience stores, and larger office buildings. These applications have been achieved by using water chillers, ice thermal storage units, and district cooling systems. In Europe, where regulatory regimes have encouraged new applications, ammonia refrigeration systems are used safely for air conditioning in hospitals, public buildings, airports, and hotels. Ammonia refrigeration provides air conditioning for the International Space Station and Biosphere II. Installation at power generation facilities represents an emerging application of ammonia refrigeration.
Environmental Advantages
What are the overall advantages of using ammonia as a refrigerant?
As a refrigerant, ammonia offers three distinct advantages over other commonly used industrial refrigerants. First, ammonia is environmentally compatible. It does not deplete the ozone layer and does not contribute to global warming. Second, ammonia has superior thermodynamic qualities, as result ammonia refrigeration systems use less electricity. Third, ammonia's recognizable odor is its greatest safety asset. Unlike most other industrial refrigerants that have no odor, ammonia refrigeration has a proven safety record in part because leaks are not likely to escape detection.
Does ammonia harm the ozone layer?
No. Ammonia does not harm atmospheric ozone. Ammonia is a natural refrigerant. It is not a halocarbon like many of the synthetic refrigerants on the market. When halocarbons are released into the atmosphere, they eventually reach the stratosphere and the ozone layer. Halocarbons are extremely stable chemically with estimated life cycles of two to three centuries. When released into the atmosphere, this stability allows halocarbons to migrate through the troposphere and into the stratosphere. At this altitude, the intense ultraviolet rays of the sun break down halocarbon molecules, releasing chlorine ions, which in turn act as catalysts to break down ozone molecules. This process reduces the ozone layer's effectiveness as a filter against ultraviolet radiation, resulting in higher amounts of ultraviolet radiation reaching the surface of the earth with harmful biological consequences. Increased radiation causes increased health risks in humans and damages the flora and fauna of the ecosystem.
Does Ammonia contribute to Global warming?
No. Just as ammonia does not damage atmospheric ozone, ammonia, with a life cycle in the atmosphere of less than one week, does not contribute to the greenhouse effect responsible for global warming. Global warming results from the short wave, near infra-red radiation that reaches the earth from the sun. About fifty percent of the sun's radiation reaches the earth. This is absorbed by the earth's surface which re-emits the radiation in longer, far infra-red wavelengths. This re-emitted radiation is partially absorbed by gasses known as greenhouse gasses. Greenhouse gasses are either natural (CO2, water vapor, etc.) or man-made (CO2, N2O, CH4, CFC, HCFC, HFC, etc.).
Is ammonia a potential substitute for refrigerants that contribute to global warming and the erosion of the ozone layer?
The Clean Air Act Amendments of 1990 gave statutory recognition to the Montreal Protocol's phase-outs in the United States and established a comprehensive set of regulatory requirements for recovery, recycling, and disposal of CFCs when equipment containing them is serviced or discarded. Part of the regulations established a U.S. EPA program for the control or phase-out of substances harmful to the stratospheric ozone layer. Through the Significant New Alternatives Policy (SNAP) program, the agency identified ammonia as an acceptable substitute to ozone depleting substances in the major industrial sectors, including refrigeration and air conditioning.
Economic Advantages
What are the overall advantages of using ammonia as a refrigerant?
As a refrigerant, ammonia offers three distinct advantages over other commonly used industrial refrigerants. First, ammonia is environmentally compatible. It does not deplete the ozone layer and does not contribute to global warming. Second, ammonia has superior thermodynamic qualities, as result ammonia refrigeration systems use less electricity. Third, ammonia's recognizable odor is its greatest safety asset. Unlike most other industrial refrigerants that have no odor, ammonia refrigeration has a proven safety record in part because leaks are not likely to escape detection.
Does ammonia refrigeration help reduce my food bill?
Generally speaking, ammonia refrigeration systems cost 10-20% less to install than systems using competitive industrial refrigerants. Thermodynamically, ammonia is 3-10% more efficient than competitive refrigerants; as a result ammonia systems use less electricity than competitive refrigerants. The cost of ammonia itself is significantly less than competitive industrial refrigerants and less ammonia is also generally required to do the job than other industrial refrigerants. All of that ad up to lower operating costs for food processors and cold storage facility operators, and that means lower grocery bills for the average household.
Do lower energy demands benefit the environment?
Proper environmental impact assessment of refrigerants and their systems requires consideration of both their direct and the indirect contribution to global warming. Refrigeration systems directly contribute to global warming through the greenhouse gas effect of their fugitive refrigerant emissions. They indirectly add to global warming through carbon dioxide emissions resulting from conversion of fossil fuels to energy required to operate the systems.
The "total equivalent warming impact," TEWI, is defined as the sum of these direct and indirect contributions. Ammonia's TEWI score is very low because ammonia itself does not contribute to global warming. In addition, due to highly favorable thermodynamic properties, ammonia refrigeration systems require less primary energy compared to other commonly used refrigerants. As a result, there is an indirect global warming benefit of lower CO2 emissions from electric power plants; among the lowest of all refrigerants.
System Safety
Are ammonia refrigeration systems safe?
In any mechanical refrigeration system, leaks will occur. This fact is exacerbated when the leaks involve odorless refrigerants as evidenced by the abundant supply of CFCs in the atmosphere today. The inherent safety of ammonia refrigeration is explained in part by ammonia's characteristic odor, which signals even the smallest leak, at concentrations far lower than any dangerous level. Ammonia's other physical characteristics such as its density and limited range of flammability, engineering advances for refrigeration systems, and the solid record of well-trained ammonia refrigeration systems operators all contribute to ammonia's excellent safety record.
Is ammonia dangerous because it smells so bad?
Ammonia's strong pungent odor gives it a self-alarming quality. The fact that it does smell so bad is one of its greatest safety assets. Even the slightest traces of ammonia in the air can be detected. This allows for the safe and immediate repair of system leaks or sources of leaks. Contrasted to the penetrating odor of ammonia, other commonly used refrigerants like the halocarbons are odorless and their escape difficult to detect without mechanical systems. The pungent odor of ammonia encourages individuals to leave the immediate area of a release before a harmful concentration builds up.
Is ammonia explosive?
Pure ammonia is difficult to ignite and has a very narrow range of flammability. Ammonia is flammable only at high concentrations and under extremely limited conditions. Ammonia vapor that contains oil or another flammable contaminant can increase the possibility of an explosion. However, ammonia will not sustain a flame on its own; ignition of ammonia vapor requires an uninterrupted external flame source.
References
http://www.aboutammoniarefrigeration.com/